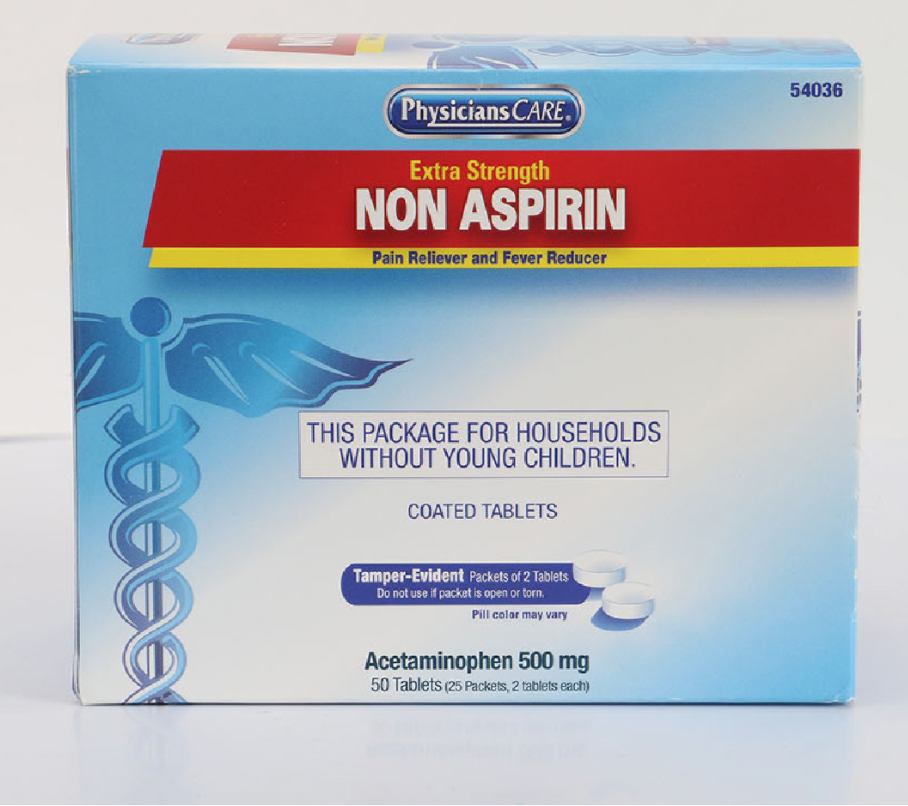
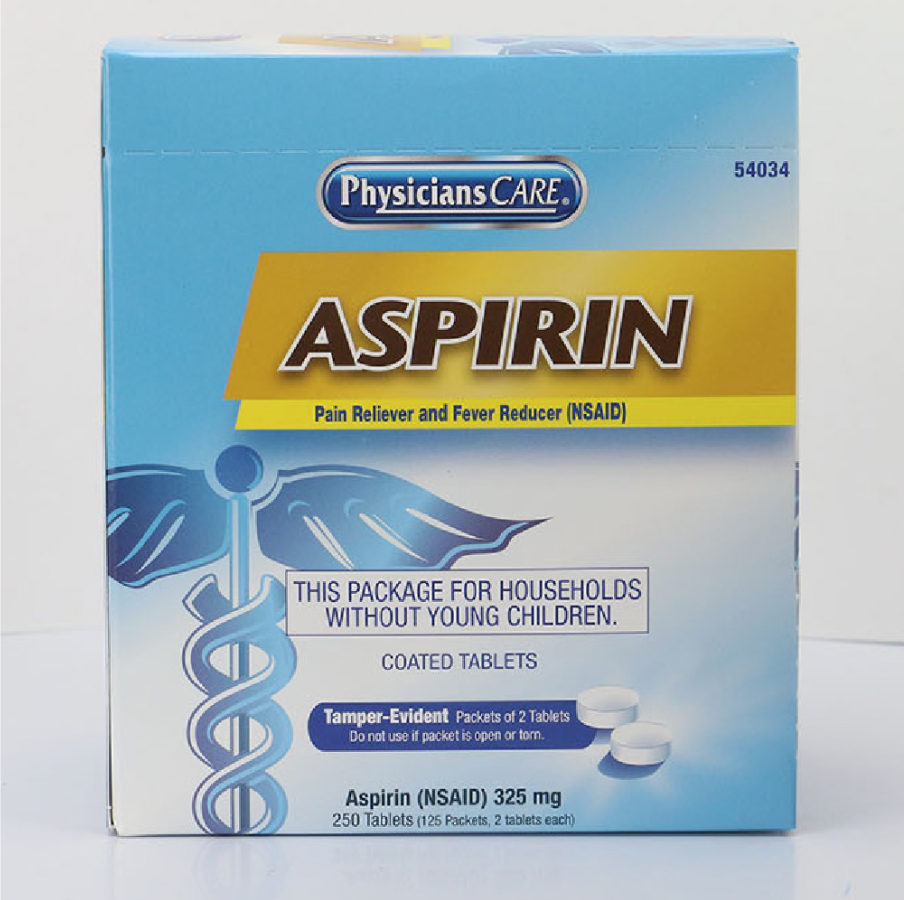
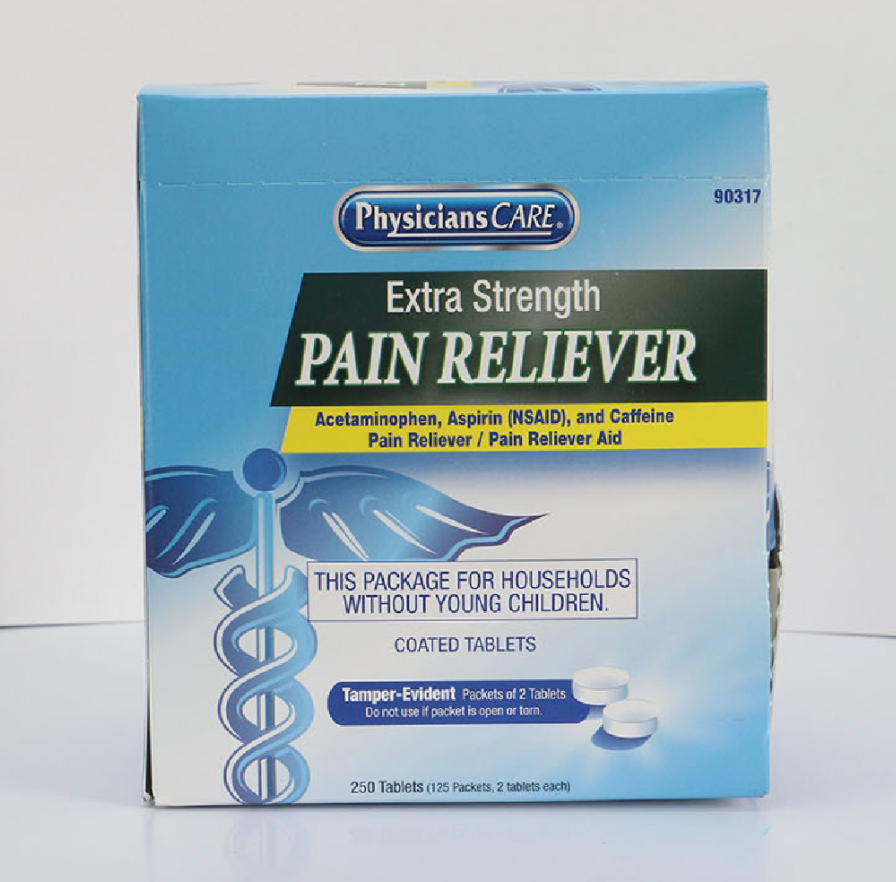
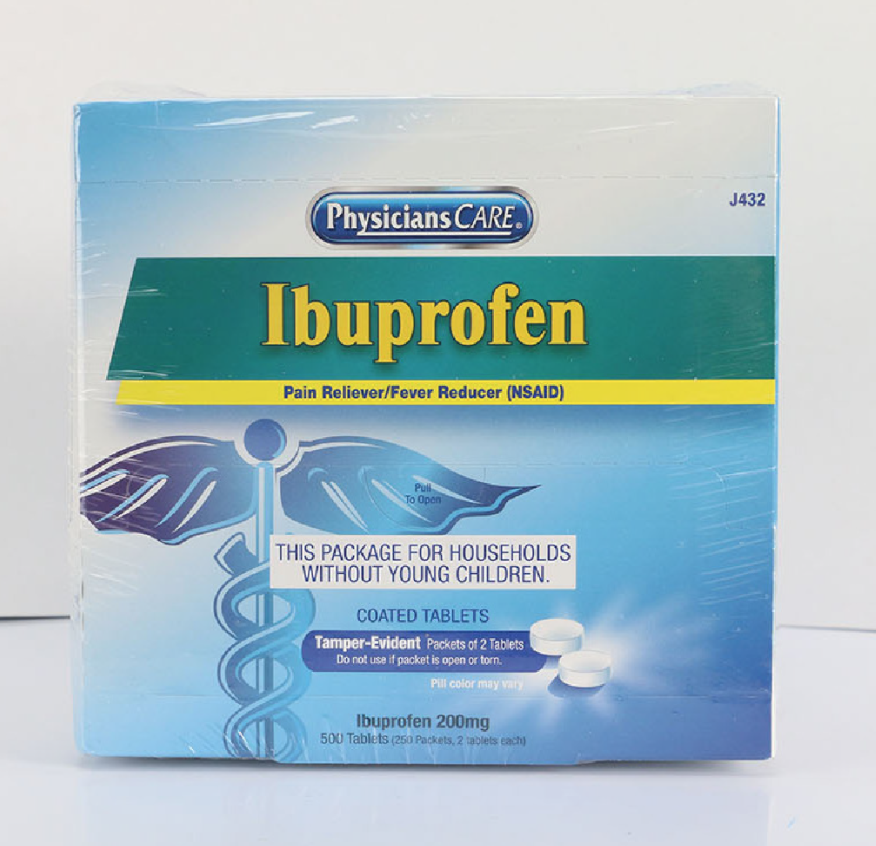
PhysiciansCare brand Aspirin, Extra Strength Non Aspirin, Extra Strength Pain Reliever, Ibuprofen, Medication Station, and Multi-Pack
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 165,000)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Acme United of Rocky Mount, North Carolina
- Reason for Recall:
- The recalled over-the-counter products contain regulated substances (aspirin, acetaminophen, or ibuprofen) which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The recalled over-the-counter products contain regulated substances (aspirin, acetaminophen, or ibuprofen) which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately store the recalled products in a safe location out of reach of children and contact Acme United for information on how to dispose of or return the product and receive a full refund. Acme United is contacting all purchasers directly.
Product Images




Product Information
Full Description:
This recall involves the PhysiciansCare brand Extra Strength Non Aspirin, Aspirin, Extra Strength Pain Reliever, Ibuprofen, Medication Station, and Multi-Pack over-the-counter drugs. The products contain aspirin, acetaminophen, or ibuprofen. They are packaged in cardboard boxes of 50, 100, 250, and 500 tablets per box. Product Drug Tablet Amount Extra Strength Non Aspirin Acetaminophen (500 mg) 50 tablets 100 tablets 250 tablets 500 tablets 2 boxes of 100 tablets each Aspirin Aspirin (325 mg) 50 tablets 100 tablets 250 tablets 500 tablets Extra Strength Pain Reliever Acetaminophen (250 mg) Aspirin (250 mg) 100 tablets 250 tablets Ibuprofen Ibuprofen (200 mg) 100 tablets 250 tablets 500 tablets 2 boxes of 100 tablets each Medication Station / Multi-Pack Acetaminophen (500 mg) Aspirin (325 mg) Ibuprofen (200 mg) Antacid (420 mg) 4 boxes of 100 tablets each with outer station 4 boxes of 100 tablets each without outer station The Antacid is not subject to this recall.
Product Codes/Lot Numbers:
(About 165,000)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 22733
Related Recalls
PhysiciansCare Allergy, Allergy Plus and Cold and Cough
Acme United, of Rocky Mount, North Carolina
The recalled products contain diphenhydramine hydrochloride and acetaminophen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.