Aprepitant capsules and Lidocaine and Prilocaine cream
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 156,750)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Sandoz Inc., of Princeton, New Jersey (Lidocaine and Prilocaine cream)
- Reason for Recall:
- The recalled prescription drugs and products that contain lidocaine must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The recalled prescription drugs and products that contain lidocaine must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately secure the medications out of the sight and reach of children and contact Sandoz for a free child resistant pouch to store the products. Once the medication is secured, consumers can continue to use the medication as directed.
Product Images



Product Information
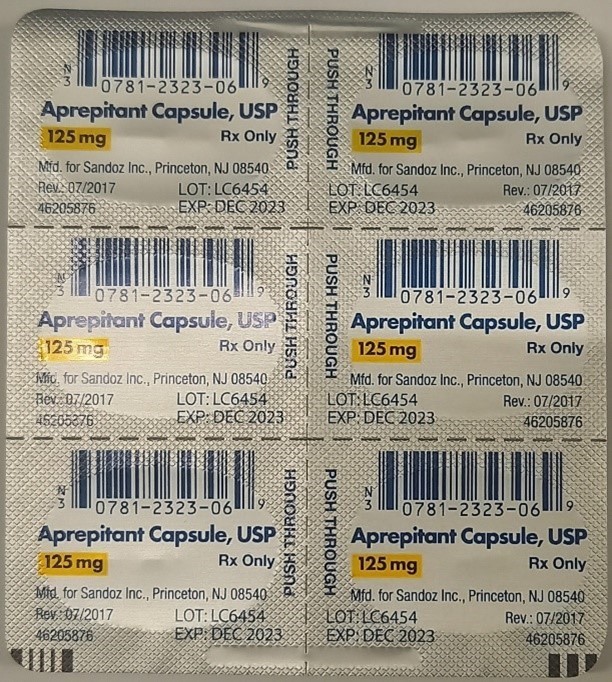
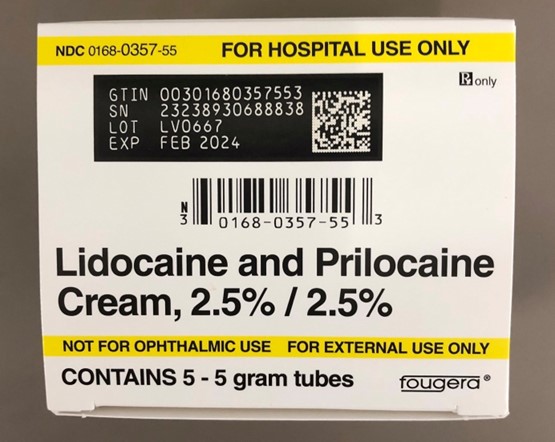
Full Description:
This recall involves prescription drugs Aprepitant 125 mg capsules sold in cartons containing one blister card of 6 capsules and 5 gram tubes of Lidocaine and Prilocaine cream sold in cartons containing 5 tubes and packed with or without 12 dressings. The Aprepitant capsules are in a non-child resistant blister card packaged in a carton that has the name "Sandoz," the name of the medication, dosage, NDC number, lot number, and expiration date on the carton and on the blister cards. The warnings "This unit-dose packaging is not child-resistant" and "For institutional use only" are listed on the carton. The Lidocaine and Prilocaine cream is packaged in a 5 gram tube with a continuous thread white closure. The name "fougera®," the name of the medication, dosage and NDC number are printed on the carton and tube and the expiration date and lot number are printed on the carton and stamped on the crimp of the tube. The warning "FOR HOSPITAL USE ONLY" is printed on the carton and the tube. Product Description NDC Number Lot Number Expiration Date Aprepitant Capsules 125 mg 0781-2323-68 Carton of 1 Blister Pack of 6 capsules 0781-2323-06 Blister Pack LK3209 LC6454 04/2024 12/2023 Lidocaine and Prilocaine 2.5%/2.5% Cream 5 gram Tubes 0168-0357-56 Carton of 5 tubes and 12 dressings 0168-0357-55 Carton of 5 tubes 0168-0357-05 Tube LA2782 LA2784 LV0667 LX5350 MA1640 MB3205 LA2785 LR9041 MB3209 03/2023 03/2023 02/2024 03/2024 03/2024 04/2024 03/2023 11/2023 04/2024
Product Codes/Lot Numbers:
(About 156,750)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 23146