Bariatric Fusion Iron Multivitamin Bottles
Class II - Moderate 🏠 Consumer Products
Recalled: September 11, 2025 VitaQuest International, LLC of Caldwell, New Jersey Clothing & Accessories
Nationwide
What Should You Do?
- Check if you have this product: (About 4,700)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- VitaQuest International, LLC of Caldwell, New Jersey
- Reason for Recall:
- The recalled multivitamins contain iron, which must be in child-resistant packaging, as required by the Poison Prevention Packaging Act. The recalled packaging of the iron-containing multivitamins violates the federal standard for child-resistant packaging because the bottle caps are not child-resistant, posing a risk of deadly poisoning, if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The recalled multivitamins contain iron, which must be in child-resistant packaging, as required by the Poison Prevention Packaging Act. The recalled packaging of the iron-containing multivitamins violates the federal standard for child-resistant packaging because the bottle caps are not child-resistant, posing a risk of deadly poisoning, if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately secure the recalled bottles out of sight and reach of children, and contact Blueroot Health for information on how to obtain a free child-resistant replacement cap.
Product Images


Product Information
Full Description:
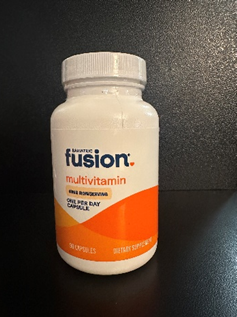
This recall involves two types of Bariatric Fusion dietary supplement bottles: high ADEK multivitamin capsules (90 and 270-count bottles) and One Per Day bariatric multivitamin capsules (90-count bottle), both with 45mg of iron. The recalled bottles are white and orange. Only bottles with smooth cap tops that lack the "push down & turn" embossed lettering are included in this recall. The Bariatric Fusion logo is printed on the front of the bottles. Lot number 0066J4, 0065J4, 0453B5 or 0370B5 is printed on the bottom of the bottles.
Product Codes/Lot Numbers:
(About 4,700)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 25460